Tag: iso 13485 training
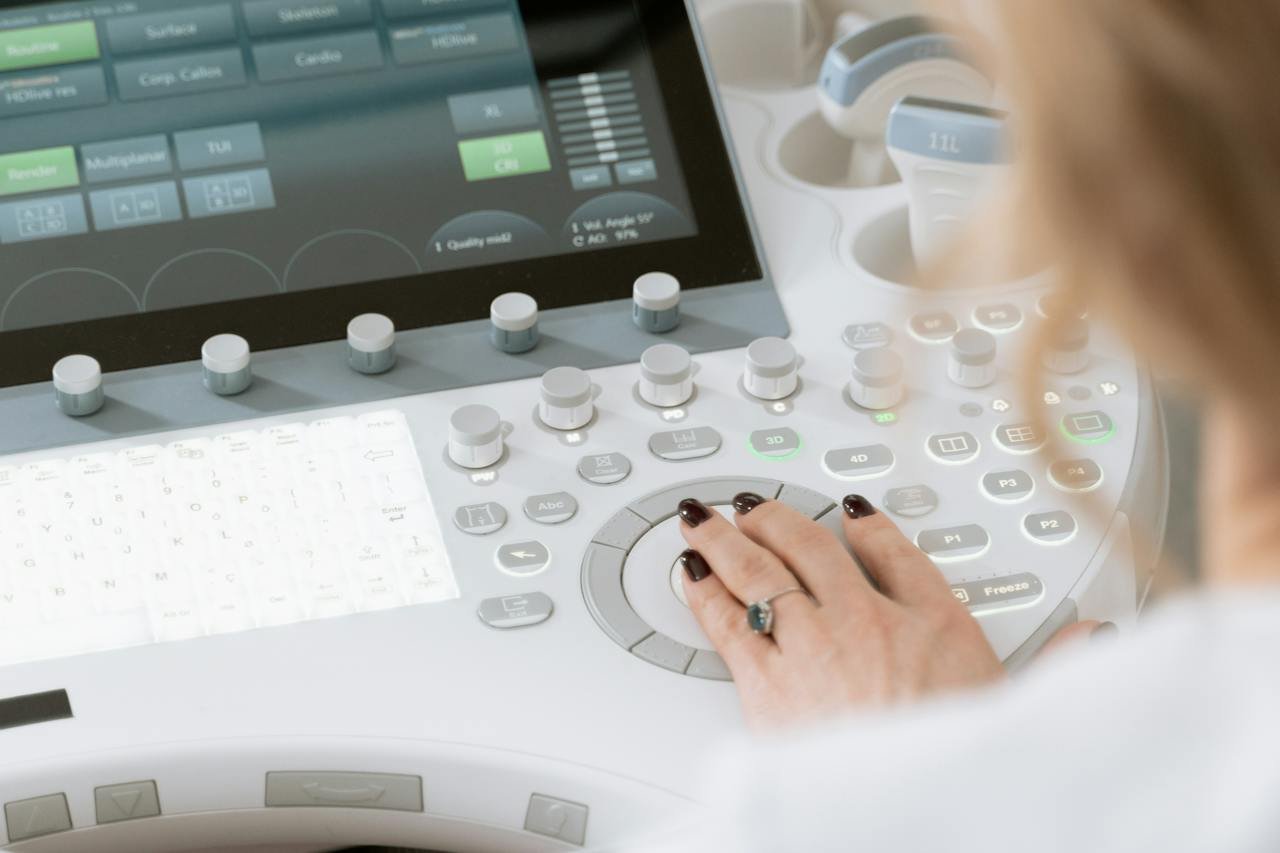
Quality Assurance in Healthcare: The Significance of ISO 13485 Medical Device Certification
The medical and healthcare industry is sensitive. One can spot special rules, norms, regulations, and certifications for healthcare operations and medical accessory production. The norms are applicable and indispensable for quality maintenance in the medical devices industry. The framework of the certification aims towards maintaining premium quality and safety. Also, it is critical to meet the compliance needs. The ISO 13485 certification offers an international-level framework to assure quality. It delivers product quality assurance to the industrial leaders and boosts the overall production rate. The best part is that you can maintain the norms and uplift the quality at every stage of manufacturing. All these help the production of high-quality medical devices. Implementing ISO 13485 fetches more than one benefit for your organization. ISO 13485 Certification – At a glance ISO 13485 is a one-of-a-kind Quality Management System standard. It is a customized framework for medical devices. The standards derive from the globally acknowledged and widely-accepted ISO 9000 QMS series. The ISO 13485 certification assists medical device manufacturers across the globe by drafting a QMS. The efficient QMS creates and maintains the effectiveness of the operations and processes. It ensures the best manufacturing design, development, production, installation, distribution, and disposal of medical devices with maximized safety and quality. The ISO 13485 standard delivers a planned structure and realistic solution that the manufacturers can adhere to. One can obtain medical device directives, regulations, development protocols, and responsibilities with the best framework. The standard helps you meet the medical device quality commitment regulations and boosts the safety and quality of medical devices. The ISO 13485 certificate could be obtained by any company manufacturing medical devices regardless of the size or location. The ISO 13485 certification is valid for all countries. Decode the significance of the certification. Obtaining the ISO 13485 certification is an integral achievement for any medical device manufacturer. The certification defines the best quality management systems and norms ensuring the safety, effectiveness, and reliability of medical devices. The ISO 13485 standard is an efficient resource designed for manufacturers of medical devices. Discover the significance of the certification to make the best choice. 1. Compliance with global norms ISO 13485 certification offers an excellent framework for manufacturers, and it eases the adherence requirements. The manufacturers can meet the regulatory requirements with a systematic approach. The norms ensure that the medical device products are safe and highly functional. The quality index also assures global recognition as an approved manufacturer of medical devices. The certification helps ensure that manufacturers can comply with the essential international standards without investing extra resources. 2. Enhanced efficiency and productivity Having an efficient quality management system can improve productivity. It plays an integral role by streamlining diverse procedures related to the manufacturing, distribution, and servicing of medical and healthcare products. It also covers the implementation steps for waste reduction and error management. One can also reduce the cost, resources, and time while focusing on customer satisfaction enhancement. The ISO 13485 standard mandates product traceability throughout the production steps. Thus, you get a chance to reduce product recalls due to noncompliance. 3. Enhanced brand reputation By obtaining the ISO 13485 certification, you can increase the brand value and attract potential customers for your company. It reflects that the company is committed to excellence, and it helps maintain the ideal practices for producing safe products with optimal functionality. The efficient QMS enhances the brand reputation in the industry and helps maintain the best professional rapport with potential business partners. Make the best decision by following strict guidelines while manufacturing medical devices. The enhanced brand recognition boosts sales opportunities amplifying customer loyalty over time. 4. Reduced hassles and recalls Product recalls are costly. You can avoid it by getting certified and following the QMS norms. It is a prime benefit of the ISO 13485 certification. Ensure the easiest way to reduce the risk of recalls caused due to safety failures. It can protect customers and organizations from hefty legal penalties and reputational damages. Get a chance to build trust among customers by exhibiting your commitment to delivering safe and high-quality healthcare products. Ensure better production and operation With the ISO 13485 certification, a company can remain focused and work towards maintaining quality goals. The management can work with essential data and make the best choices. These information pieces can align better and help you make strategic moves for achieving organizational objectives. It is a proactive solution and helps in the continual improvement of the QMS. It is one of the paramount aspects of the ISO quality management systems. All these benefits help the organization in more than one way, improving the workforce culture. Customer satisfaction and employee involvement – The ultimate need Customer satisfaction is among the critical aspects of ISO 13485. The ISO-certified products are premium and reliable for the customers. Hence, they can use the products without second thoughts. It develops a robust professional bond, exhibiting the best brand value for the company. Boost the productivity quotient with ample opportunities for QMS improvement and follow the ideal norms of ISO certification and framework. Summing up The ISO standard facilitates the operations of manufacturing companies and helps them reduce safety and law-related risks. It also creates an economical work environment. With the internationally recognized standard, ensure an efficient and hassle-free solution for the quality and safety of medical device manufacturing. Follow a systematic process to obtain the ISO 13485 certification and get recognized as a reputable and trustworthy brand in the competitive market. Connect to IRQS, the trusted partner for audit requirements. Get in touch with expert auditors with years of experience and expertise to obtain accurate audit reports. Find the best-in-class audit resources with the service experts and resolve the requirements efficiently.
Search
Useful Links
Recent Posts
What Is Carbon Footprint Verification & Why It’s Critical for Your ESG Goals

Why ESG Reporting Is the Future of Business Strategy



